Praesent sapien massa, convallis a pellentesque.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Proin eget tortor risus. Pellentesque in ipsum id orci porta dapibus. Mauris blandit aliquet elit, eget tincidunt nibh pulvinar a. Vestibulum ante ipsum primis in faucibus orci luctus et ultrices posuere cubilia Curae; Donec velit neque, auctor sit amet aliquam vel, ullamcorper sit amet ligula. Nulla quis lorem ut libero malesuada feugiat.
Septiflo
Septiflo is a patent-pending first and only rapid card test available globally for endotoxin detection in a POC format. Lipopolysaccharide endotoxins are released very early into the bloodstream and trigger Gram-negative bacterial sepsis. Similarly, lipoteichoic acid is a major trigger for Gram-positive bacterial sepsis. Endotoxin detection by special laboratory-based luminescence tests and removal of endotoxin from blood by hemoperfusion are already used in sepsis management in tertiary care hospitals. We have developed two devices, Septiflo-N for Gram-negative infections and Septiflo-P for Gram-positive infection detection. Both these devices give semi-quantitative colorimetric estimates of the extent of infection in blood in less than 10 min in a simple and elegant manner.
Technology Impact
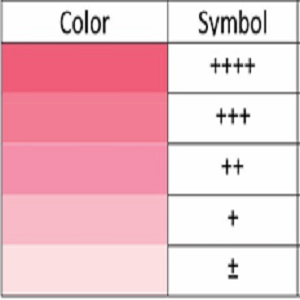
Mortality in sepsis is very high in critical-care patients and the major reason for this is delay in pathogen diagnosis. Every hour of delay decreases the survival by 8 to 10 % and this is where our technology and device adds value. Our device helps in not only rapid diagnosis of bacterial bloodstream infections very early into the infection but can also assess indirectly the severity of infection by correlating to the biomarker concentration. It also gives Gram status of the bacterial infection within a few minutes, improving evidence-based therapeutic decision time and monitoring of treatment response. The low-cost of the device also makes it more accessible to low and middle income groups, thereby having a dramatic and positive impact on the outcome of sepsis management in intensive care units around the globe. It is also hoped that use of our devices would help to reduce development of antibiotic resistance. All the technologies currently available require time for growth in culture and detection by sophisticated technologies which involve expensive laboratory based instruments and protracted time to diagnosis. Our devices are simple to use and can be operated by a semi-skilled worker. The results are also fairly easy to interpret. This is done using a colour chart (shown below on the left) and matching its colour band with the spot intensity obtained on the device after running the assay (shown below on right). The spot’s colour intensity correlates to the severity of infection.

Publications
Journal of Clinical Microbiology, 56(9), 1-13 (2018)
Jagtap P., Singh R., Deepika K., Sritharan V. and Gupta S.
Molecular Systems Design and Engineering, 2 ,470-477 (2017)
Kalita P., Bhola A., Goel N., Sritharan V. and Gupta S.
Nanomedicine: Nanotchnology, Biology, and Medicine, 13, 1483-1490 (2017)
Kalita P., Chaturvedula M., Sritharan V. and Gupta S.
Analytical Chemistry, 87, 11007-11012 (2015)
Kalita P., Dasgupta A., Sritharan V. and Gupta S.
Patents
Kalita P., Sritharan V. and Gupta S. “An Assay and Kit for Detection of Endotoxin”, Indian Patent 201611007932 (PCT/IN2016/050397); US Patent publication no. 2019/0079088 (filed Sep. 06, 2018); EU Patent App. 16893365.3 (filed Oct. 01, 2018)
Gupta S., Kalita P., Dasgupta A. and Sritharan V., “Platform Device and Method for Detection of Bacterial Lipolysaccharides in Water and Serum”, Indian Patent 3214/DEL/2014

